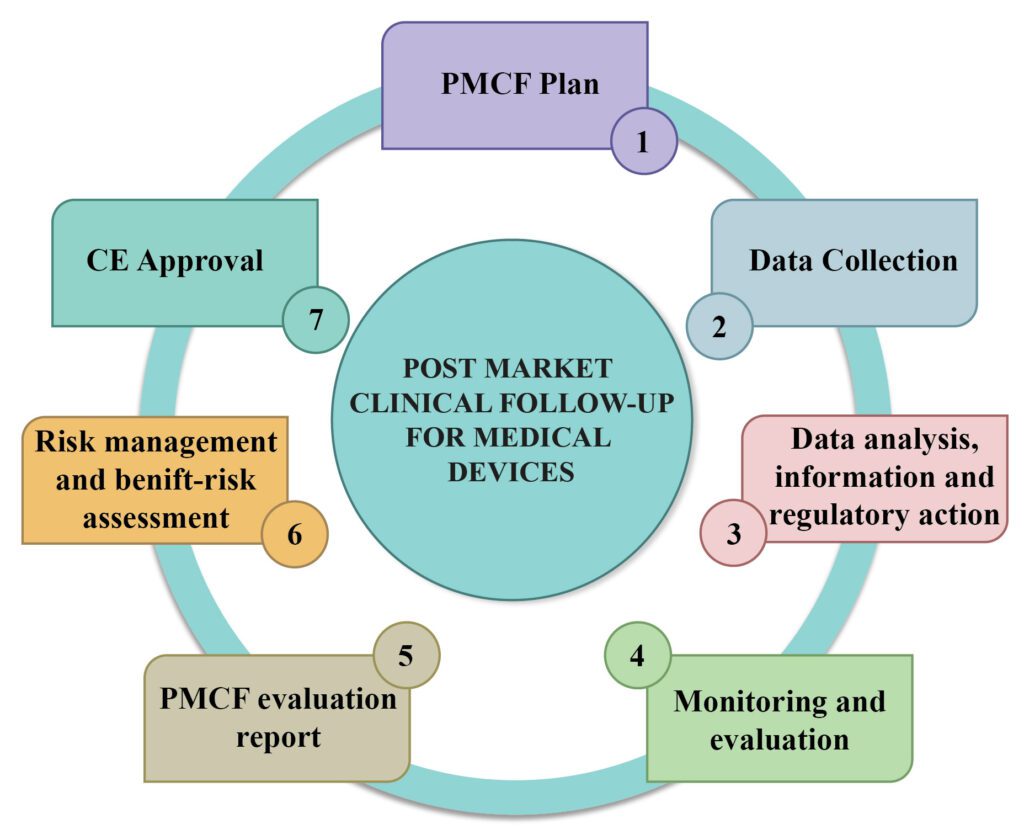
Post market clinical follow-up and Post-market surveillance (PMS) refers to the monitoring of medical devices after they have been cleared for sale and are in use by the public. One of the several types of post-marketing surveillance on the use of medical devices stipulated by the European Union Medical Device […]