Regulatory Affairs in Action: Recent FDA Drug Approvals and Medical Breakthroughs
Regulatory Affairs is fundamental in shepherding the groundbreaking treatments through the approval process, ensuring that they meet rigorous standards for safety and efficacy. Each year, the United States Food and Drug Administration (FDA) receives a considerable number of New Drug Applications (NDAs) and Biologic License Applications (BLAs). However, from 2011 to 2020, the FDA granted an average of approximately 40 approvals for novel drugs annually, resulting in a total of only 410 therapies being introduced to the market during that timeframe. The world of pharmaceuticals has witnessed extraordinary progress in recent years, with the introduction of several FDA-approved medications revolutionizing the treatment of various conditions.
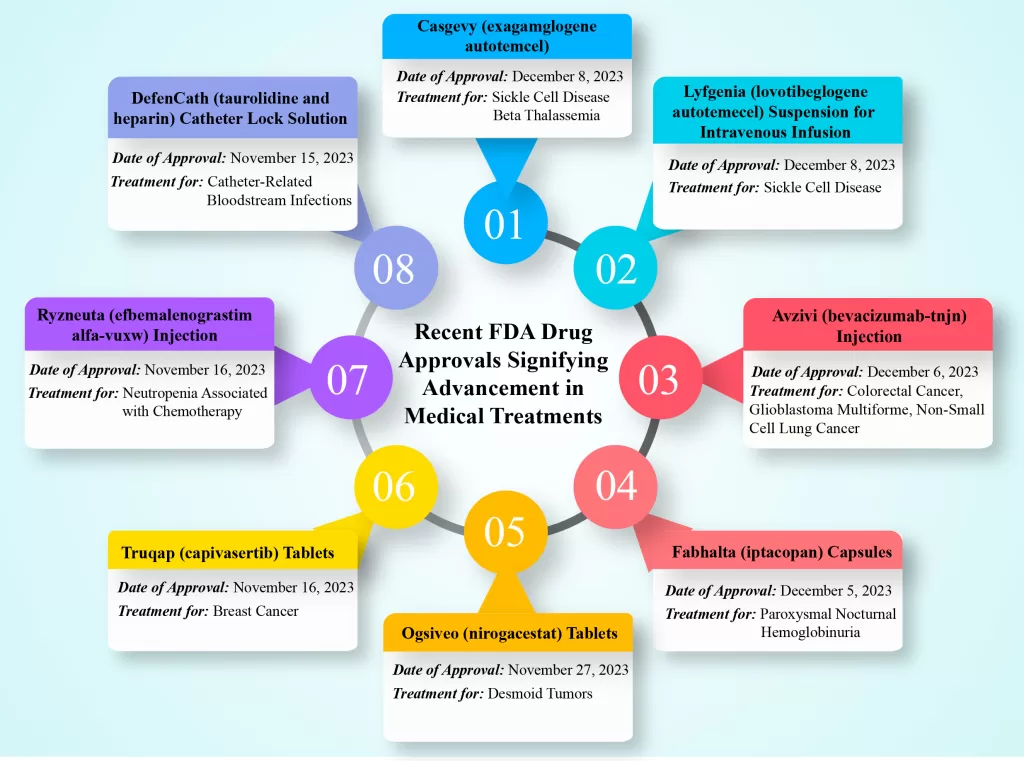
Recent FDA Drug Approvals Signifying Advancement in Medical Treatments:
The FDA has recently approved several groundbreaking medications, showcasing significant progress in diverse therapeutic areas. From gene-edited therapies to innovative cancer treatments, these approvals open new avenues for medical advancements.
- Casgevy (exagamglogene autotemcel)
- Company: Vertex Pharmaceuticals Incorporated
- Date of Approval: December 8, 2023
- Treatment for: Sickle Cell Disease, Beta Thalassemia
Casgevy, a CRISPR/Cas9 gene-edited therapy, marks a crucial step in treating sickle cell disease (SCD). The therapy utilizes autologous genome-edited hematopoietic stem cells, addressing recurrent vaso-occlusive crises (VOCs) in patients aged 12 and older.
- Lyfgenia (lovotibeglogene autotemecel) Suspension for Intravenous Infusion
- Company: Bluebird Bio
- Date of Approval: December 8, 2023
- Treatment for: Sickle Cell Disease
LYFGENIA™ (lovotibeglogene autotemcel) is a one-time gene therapy designed by Bluebird Bio for patients aged 12 and older with a history of vaso-occlusive events (VOEs). This custom-made therapy aims to resolve VOEs, addressing the root cause of SCD.
- Avzivi (bevacizumab-tnjn) Injection
- Company: Bio-Thera Solutions, Ltd.
- Date of Approval: December 6, 2023
- Treatment for: Colorectal Cancer, Glioblastoma Multiforme, Non-Small Cell Lung Cancer, Renal Cell Carcinoma, Ovarian Cancer, Fallopian Tube Cancer, Cervical Cancer, Peritoneal Cancer
Avzivi, a biosimilar to Avastin, is a vascular endothelial growth factor inhibitor approved for treating various cancers.
- Fabhalta (iptacopan) Capsules
- Company: Novartis Pharmaceuticals Corporation
- Date of Approval: December 5, 2023
- Treatment for: Paroxysmal Nocturnal Hemoglobinuria
Fabhalta (iptacopan) stands out as a first-in-class complement factor B inhibitor, providing a treatment option for adults with paroxysmal nocturnal hemoglobinuria.
- Ogsiveo (nirogacestat) Tablets
- Company: SpringWorks Therapeutics, Inc.
- Date of Approval: November 27, 2023
- Treatment for: Desmoid Tumors
Ogsiveo (nirogacestat), a gamma secretase inhibitor, offers hope to adult patients with progressing desmoid tumors requiring systemic treatment.
- Truqap (capivasertib) Tablets
- Company: AstraZeneca
- Date of Approval: November 16, 2023
- Treatment for: Breast Cancer
Truqap (capivasertib), an AKT inhibitor used in combination with fulvestrant, provides a novel approach to treating advanced hormone receptor-positive breast cancer.
- Ryzneuta (efbemalenograstim alfa-vuxw) Injection
- Company: Evive Biotech
- Date of Approval: November 16, 2023
- Treatment for: Neutropenia Associated with Chemotherapy
Ryzneuta (efbemalenograstim alfa-vuxw) is a leukocyte growth factor, significantly reducing the duration of febrile neutropenia in patients undergoing chemotherapy for certain cancers.
- DefenCath (taurolidine and heparin) Catheter Lock Solution
- Company: CorMedix Inc.
- Date of Approval: November 15, 2023
- Treatment for: Catheter-Related Bloodstream Infections
DefenCath (taurolidine and heparin) catheter lock solution is a unique combination that reduces the incidence of catheter-related bloodstream infections in adult patients with kidney failure receiving chronic hemodialysis through a central venous catheter.
Conclusion
Regulatory Affairs play a pivotal role in shaping the landscape of medical advancements, particularly in the context of regulatory drug approvals. These drug approvals hold immense promise for improving patient outcomes and addressing critical healthcare needs. These recent FDA drug approvals underscore the relentless pursuit of innovation in the pharmaceutical industry, offering hope and improved treatment options for patients facing diverse health challenges. These approvals mark significant milestones, opening avenues for the development of novel therapies and presenting new hope for treating various diseases.
Navigating the intricate landscape of regulatory drug approvals requires expertise and precision, and that’s where WorkSure® excels in providing unparalleled Regulatory Affairs services. With a proven track record in conducting clinical trials, including those involving novel drugs/devices/biologics, WorkSure® ensures a seamless journey through the regulatory process. Collaborating with WorkSure® means entrusting your drug development process to a team dedicated to ensuring scientific rigor and compliance, ultimately increasing the likelihood of securing regulatory approvals.