About WorkSure®
A Peek into the Organization

WorkSure®, a Contract Research Organization (CRO) and Medical Affairs (MA) company, stands as a beacon of promise within the healthcare industry, ceaselessly propelling towards progress and pioneering innovations. The company offers specialized scientific support to the indirect patient care segment, bridging the gap between academia and the healthcare industry and improving the overall effectiveness of direct patient care interventions. Apart from this, WorkSure® provides medical affairs and clinical trial management services to a wide range of stakeholders including hospitals, pharmaceutical and medical device companies along with government institutions. We deliver comprehensive support while catering to the client’s requirements, further assisting our customers in improving and accelerating the delivery of novel products and therapeutics that improves health globally.
Since 2010, we have had an excellent outcome and provided unique solutions that aided the healthcare industry in bringing scientifically validated products and therapies. We have a track record of delivering high-quality medical writing services that can boost the write-up process of pre-clinical, clinical studies, and medico-marketing activities. We work as Clinical Research, Medical Affairs & Technology Partner for multinational pharmaceuticals, nutraceuticals, biotechnology industries, medical device industries, hospitals, governmental organizations, and non-governmental organizations. We have successfully conducted and are undertaking clinical trials for both drugs and medical devices. WorkSure® has been ISO 9001:2015 certified for its constant quest for delivering quality services through its audited Quality Management Systems.
Our clinical development and integrated consulting expertise help you streamline the product development process, making the process faster and more cost-effective. From decentralized clinical trials to regulatory consulting services and utilization of real-world data, our therapeutic, functional, and technological competency adheres to a patient-centric culture. Our organization also provides strategic solutions to ensure faster timelines, better quality, and the most efficient delivery of clinical trials at every stage. Our solution-oriented services ensure that the clinical trials are executed timely and consistently as per the regulatory guidelines.
WorkSure® has emerged as one of the fastest-growing and preferred CRO and MA companies, committed to delivering outcome-based medical solutions and products. Over the last 13 years utilizing various innovative, value-added, and evidence-based services and products, WorkSure® has delivered its solutions globally to the Asia-Pacific (APAC) region, the Middle East and North Africa (MENA) region, Japan, Europe, the United Kingdom, and the United States. We use innovative technology, a firm commitment to quality, and therapeutic expertise to bend the time curve and cost of drug or medical device development.
The highly qualified and experienced team at WorkSure® facilitates collecting, organizing, editing, rearranging, and producing medical and scientific documents required for your product development. The core team comprises alumni from prestigious institutions like AIIMS, IIM, IIT, and PGIMER. Dedicated medical affairs and clinical research staff (specialist and super-specialized doctors), supported by a strong IT team and a competent workforce of medical writers, medical coders, biostatisticians, PhDs, and graphic designers have propelled our expansion.
WorkSure® works to enhance public health and clinical research in collaboration with other enterprises including government and non-government organizations. Our objective is to help our clients to become more agile and accelerate patient outcomes by providing smart and innovative solutions, connecting their needs, our competence, and the healthcare ecosystem. We strategically blend expertise, innovation, and cutting-edge technology to address modern market realities and meet customers' needs while adhering to the industry’s best practices.
Organization Overview

-
Our Logo
-
Our Vision
-
Our Mission
-
Our Values
The double tick (√√) in our logo represents the right approach. The letter S portrays our sincerity and assurance. The two dots (..) stand for our commitment to perfection and punctuality. The blue and green colors represent the overall creativity and progress of the team.

To be the most preferred medical knowledge partner, ideal for various healthcare services and technology platforms across the globe
- To redefine global medical service support and healthcare knowledge partnership by delivering effectual, pertinent service and technology platforms for clinical & commercial success- thereby bridging academia and industry
- To contribute to a healthy world and achieve exponential growth in the healthcare sector by speeding up innovations and establishing strong connections throughout the healthcare ecosystem
WorkSure® combines technology and healthcare expertise to help customers make better healthcare-related decisions and improve patient outcomes. We are committed to social, governance, and sustainable environmental practices around the globe as part of our mission to create a healthier world. Our services assist our customers in improving and accelerating product deliverables that have a global impact on health.
- Innovation: WorkSure® supports scientific discoveries through innovations, as well as through cutting-edge research and development. We pay attention to our customer’s needs and revert with creative solutions.
- Quality: Our organization values quality in all aspects of our business and focuses on every detail to get the greatest possible result. We take our work seriously, treat others with empathy, and are dedicated to making a difference. Understanding clients’ objectives, requirements, and final scope is of utmost importance.
- Integrity: Serving our partners with honesty and integrity and abiding by all applicable laws while conducting our business is our prime importance. We foster open communication during every stage of our services. We leverage customer and employee feedback to make company-wide improvements.
- Proficiency: We are deeply committed to delivering high-quality outcomes with the highest possible ethical standards within the desired time limit. To achieve the best-integrated performance, our company provides training and development opportunities for both our employees and customers. We believe that our growth and success are strongly related to the professional development of each of our employees.
- Respect: Creating an environment that focuses on mutual respect, openness, and personal integrity is our focus. We care about the well-being of everyone who interacts with us or is affected by our business. Our people-centric culture promotes and respects new ideas and invites our employees to share their diverse backgrounds.
Why WorkSure®
-
Team WorkSure®
-
Confidentiality & Data Security
A perfect blend of youthful, energetic team members with new ideas, strong academic credentials, and extensive industry experience, backed by a distinguished advisory board, bring complementary qualities to complete various projects.
- A highly qualified and skilled workforce (medical postgraduates and PhDs) with excellent language skills
- Ability to work on products at all phases of development, from pre-clinical through post-marketing/commercialization
- Expertise and proficiency in a wide range of therapeutic areas
- Resourceful and innovative when it comes to developing realistic solutions for clinical and commercial success
- Well-versed with the policies and guidelines of different international and national societies and journals
- Good understanding of national and international regulatory requirements
- Strong in-house training modules and regular updates for medical writing and data management teams
- Dedicated to providing excellent customer service and a cutting-edge technology platform
- Constantly seeking for new solutions to overcome obstacles
- Rigorous and structured policy for recruitment and selection
- Adhering to all applicable standards:
- The Food and Drug Administration (FDA) regulations
- The European Medicines Agency (EMA) regulations
- The Central Drugs Standard Control Organization (CDSCO) regulations
- The General Data Protection Regulation (GDPR) data privacy requirements
- Part 11 of Title 21 of the Code of Federal 19 Regulations (21 CFR part 11) Compliance
- Government security standards
- ISO 9001:2015 certified
Our data security procedures are constantly evolving following client needs, data protection legislation, industry best practices, and regulatory data security standards.
- Non-disclosure agreements (NDAs) with clients and employees
- Project-related IP protection
- Physical security via restricted access and a 24x7 surveillance system
- Windows and application-based intranet access control
- Application-based centralized and monitored access for external storage devices
- Restriction of web access via user-specific restrictions
- Implementation of advanced risk management methods to identify and treat hazards related to vital technological systems and sensitive data
- Adherence to the most cutting-edge technology to keep an eye on potential threats
- Risk assessments and risk management for cloud-hosted and on-premise systems, as well as vendors
- Routine internal and external control testing to assess information security procedures
- Comply with stringent government security standards and conduct frequent security audits to ensure compliance
- Employs a defense-in-depth strategy that includes multiple layers of security protection
- Conducts continuous information security training to promote awareness and knowledge about human susceptibility to cyber dangers and the proper use of technology
Our Specialities
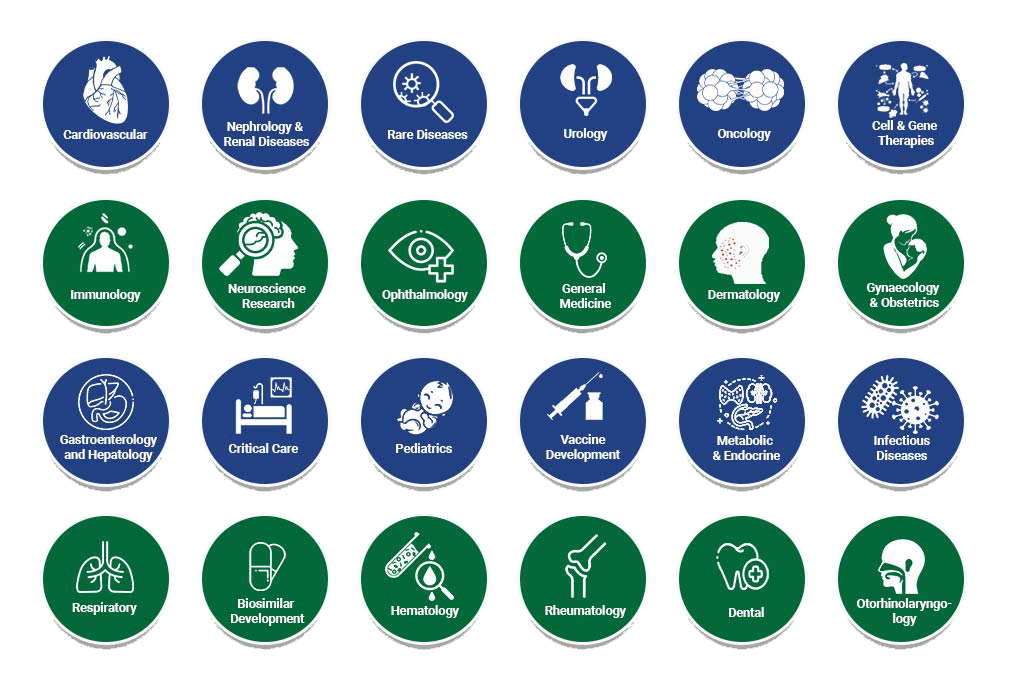
The core competency of WorkSure® lies in the medical domain, the clinical research domain, and the robust IT infrastructure, complemented by the concept of medically driven outcome-based solutions. We leverage our knowledge and experience in strategizing and conducting clinical studies in diverse therapeutic areas. The organization aims to harness excellence to provide innovative solutions in medical affairs management, clinical research management, developmental strategy, product launching, and regulatory planning & management. Our team involves experts from different therapeutic areas who have the capabilities to comprehend and overcome the obstacles you confront.
WorkSure® covers the following therapeutic areas: