Nutraceuticals in Clinical Trials: A Key to Evaluating Nutraceuticals and Dietary Supplements
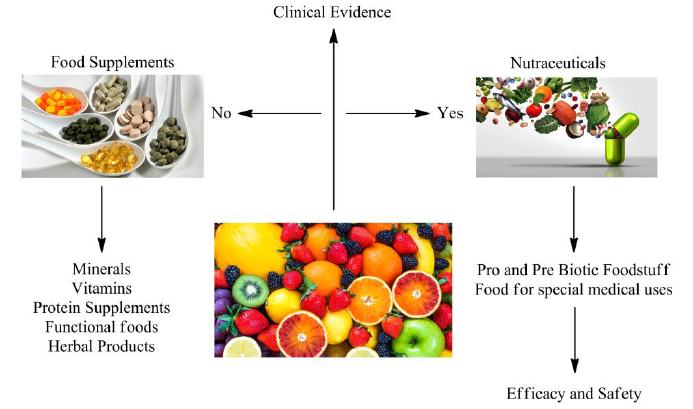
“Nutraceuticals,” a term coined by Dr. Stephen DeFelice in 1989, refers to nutritionally enriched foods and dietary supplements that are purified and formulated health products containing bioactive food compounds. These products support optimal health by supplementing the diet with herbs, vitamins, minerals, and amino acids. They are intended to enhance life expectancy, support bodily functions, and contribute to disease management. For example, omega-3 fatty acids have been shown to decrease glucose intolerance in individuals prone to diabetes. Nutraceuticals include combinations of probiotic and prebiotic foodstuffs for special medical uses, available in various forms such as capsules, tinctures, and tablets. Examples include Spirulina, Panax ginseng, lycopene, extracts from the Allium family (garlic, onion), glucosinolate extracts, and phytosterol extracts.
The nutraceutical industry has experienced significant global growth, particularly in healthcare promotion and disease prevention. This expansion is driven by increasing public awareness of the benefits of nutrition, highlighting the need for stringent regulations to prevent uncontrolled use and potential side effects. However, due to their multifunctional nature, studying their mechanisms of action, safety, and efficacy through clinical research presents challenges. The rapid growth of nutritional products and health foods necessitates clinical trials or in-vitro tests to ensure their efficacy and safety. Clinical trials assess safety, efficacy, bioavailability, and the potential to prevent side effects, with a primary focus on disease recovery.
Since nutraceuticals are classified as food, premarket safety and efficacy data are not mandatory for FDA approval, making their market entry less complex than that of pharmaceuticals. However, if a product claims to cure a disease, it should be classified as a drug. Any product intended to diagnose or mitigate a disease condition must undergo rigorous regulatory scrutiny. Manufacturers and regulatory authorities must ensure that these products are safe, contaminant-free, manufactured according to Good Manufacturing Practice (GMP) guidelines, and accurately labeled before being made available to the public.
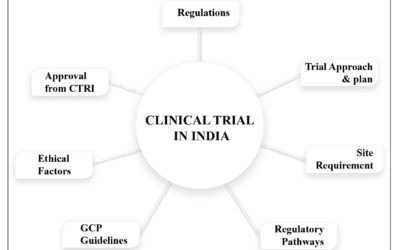
Scientific advancements necessitate standardization of constituents and protocols, along with the implementation of clinical studies to safeguard consumer health and guide nutraceutical companies. Clinical trials systematically evaluate nutraceutical performance in human subjects by verifying clinical aspects and potential adverse effects based on patient and consumer needs. From a regulatory perspective, nutraceuticals are categorized under different terms depending on the country. For example, in Canada, they fall under Natural Health Products (NHPs) and supplemented foods. The European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration (FDA) use evidence-based reviews to evaluate scientific data supporting marketing claims for dietary supplements. Defining clear and measurable clinical endpoints for nutraceuticals can be challenging due to their subtle effects, which may vary among individuals. Clinical trials in this field tend to be shorter, involve smaller sample sizes, and target broad population groups such as women, athletes, the elderly, or individuals with subclinical conditions.
Conclusion
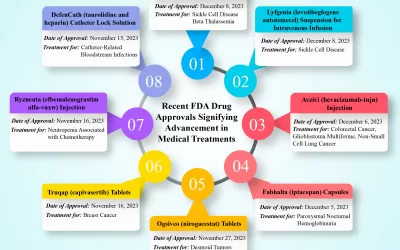
Numerous clinical and preclinical studies have demonstrated that adequate consumption of nutraceuticals and dietary supplements can help manage and prevent diseases such as cardiovascular disorders, cancer, and gastrointestinal conditions. For example, vitamin C has been studied for its role in managing various health conditions, including its potential benefits during the SARS-CoV-2 pandemic, and for reducing the risk of Alzheimer’s disease. Additionally, nutraceuticals such as resveratrol, omega-3 fatty acids, alpha-lipoic acid, curcumin, and L-arginine have been subjects of clinical trials to assess their effectiveness. Therefore, extensive evidence-based studies are needed to establish their long-term safety, efficacy, and quality.
WorkSure®, a medical affairs and contract research organization, provides comprehensive solutions for clinical trials in the field of nutraceuticals. With expertise in end-to-end trial management, WorkSure® ensures appropriate patient selection and recruitment, efficient protocol planning, medical writing support, and clinical data management, all in compliance with Good Clinical Practice (GCP) standards.