Medical Writing Principles: Scope and Opportunities
Medical writing involves crafting scientific papers by collaborating with doctors and scientists who generate the data, even if the writer isn’t the original researcher. A deep understanding of medical concepts is essential to effectively convey information in a way that the intended audience can understand.
Medical writers tailor content to suit specific guidelines and audience levels, making their role vital in the pharmaceutical industry. Proficiency in writing and medical knowledge is crucial, along with staying updated on relevant guidelines. The demand for skilled medical writers has steadily grown, offering numerous career opportunities in the pharmaceutical and healthcare sectors. Graduates and postgraduates in life sciences may consider medical writing as a viable full-time career if they have the necessary skills.
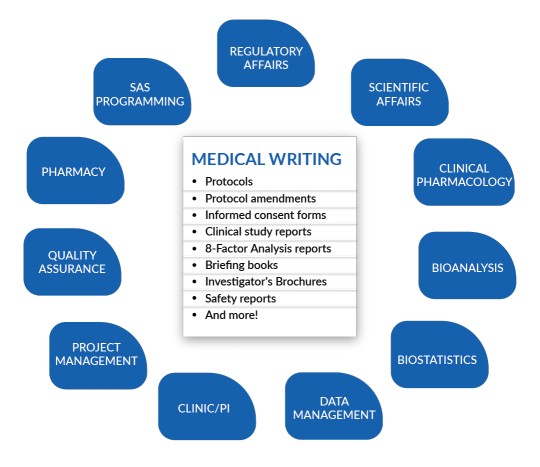
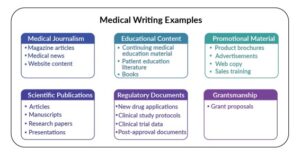
Writing various kinds of documents for various audiences and goals is a part of medical writing. Some samples of several types of medical writing are as follows:-
Medical Journalism:- Articles in newspapers and magazines should be written in plain, non-technical language since their primary audience is the general public and laypeople.
Medical Education For Physicians:- Textbooks, Continued Medical Education (CME) programs, slide decks, e-learning modules for patients (patient education material).
Publication / Presentation Journal articles / manuscripts (research articles, case reports, review articles):- Abstracts, posters and presentations for scientific meetings and conferences.
Research Documents:- Clinical trial protocols, Investigators’ Brochure, Informed Consent Documents, Study reports, Research proposals, Regulatory Documents.
Regulatory Documents:-
- Package Inserts (prescribing information) &
- Patient Information Leaflets
- Clinical study reports, web synopses
- Subject narratives
- Regulatory submission documents such as clinical overviews & summaries, expert reports, safety & efficacy summaries
The pharmaceutical sector is the primary client for medical writers.
Nonetheless, there are numerous additional contexts where medical writers are needed, including:
- Manufacturers of pharmaceuticals and other healthcare products, such as medical device manufacturers
- Contract research organizations (CROs) and business/knowledge process outsourcing firms (BPOs / KPOs)
- Corporations that provide functional service providers (FSPs) for scientific content and healthcare communication
- Medical journals and media and publishing firms
- Medical/scientific associations, academic medical institutions, and healthcare websites
Steps in writing scientific documents:
- Understanding the project brief
- Literature search & review of information
- Authoring & compiling the document
- The review process
- Formatting & editing
- Approval and sign off
- Electronic publishing
Conclusion
Each type of medical writing is meant for a distinct set of audience, e.g. medical professionals, patients & general public, medical sales representatives or drug regulators. As a result, the language employed and the degree of technical detail provided must be suitable for the audience in question. Publications intended for patients and the general public, on the other hand, must be straightforward and devoid of technical jargon, even though publications meant for medical professionals and regulators might be extremely technical and include scientific data and its explanation. Furthermore, materials submitted to regulatory bodies must adhere to specific formats and structures, and the regulations’ rules and guidelines govern their content. Therefore, a medical writer who prepares these documents must be familiar with the rules and required formats for these kinds of documents.
WorkSure® provides all-inclusive regulatory medical writing services, including the know-how and direction required to successfully navigate the regulatory environment, adhere to rules, and communicate the brand message. For expert regulatory help and to guarantee the success of your products, get in touch with WorkSure® right away.