Medical Affairs Management For Driving Improvement in Patient Outcomes
Medical affairs management identify and address the medical needs associated with products, therapies and disease states thereby driving improvement in patient outcomes in line with commercial objectives. Medical affairs came into existence in pharmaceutical and biotech industries to aid interactions between health care professionals, company representatives and the end-users. Medical affairs has transformed is a strong traditional support function for the users.
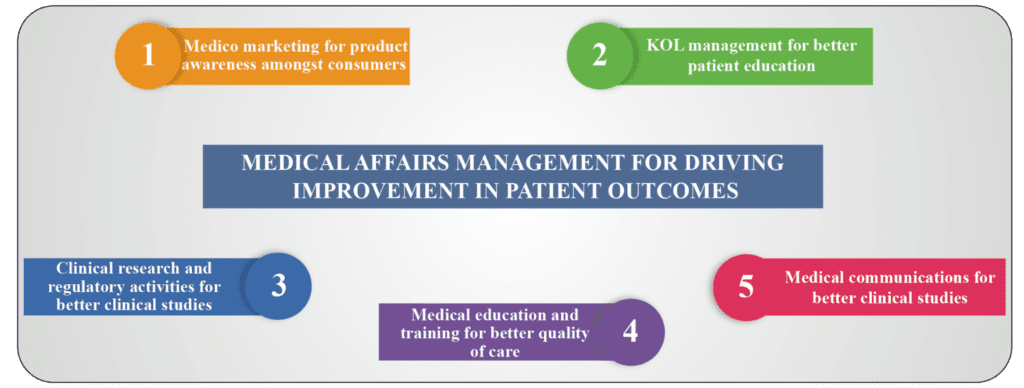
Importance of Medical Affairs Management for improvement in patient outcomes
- Medico marketing for product awareness amongst consumers
The complexity of medical marketing is one of the vital aspects of a business proposal in the pharmaceutical industry. Promotion refers to all informational and convincing activities by company and distributors, the effect of which is to bring about the prescription, supply, purchase and/or use of medicinal drugs. Medical affairs review external communications for ethical and credible promotion of all brands amongst the patients. Across product life cycle, the medical affairs provide guidance on therapy positioning in all marketing and promotional campaigns and keep a close watch on the approvals. Medical affair activities ensure appropriate market conditioning which has a real impact on increasing product awareness amongst the subjects.
- KOL management for better patient education
The communication of meaningful information like crafting scientific exchange tools and resources for continued medical education on disease state, current therapeutics, and patient care gaps is the underpinning of ongoing interaction between Key opinion leaders (KOL)s and the end-users. KOLs management should be aligned with both scientific and commercial perspectives which require optimal organizational structure. Harmonizing KOL activity is increasingly crucial for both compliance and effectiveness for quality care of the patients.
- Clinical research and regulatory activities for better clinical studies
Medical affairs team works closely with the clinical development team for planning and organizing clinical trials for better care of the patients. They support clinical development plan, protocol development, investigator selection and meetings, ongoing data maintenance and management, statistical analysis, interpretation, report writing activities etc. They are involved in pharmacovigilance, regulatory support, post-marketing safety and surveillance studies of the drug/ device administered to the subjects of the study. Medical affairs professionals evaluate and conduct pharma co-economic studies to facilitate cost effective health care decision making. The medical affairs department plays a central role in strategizing regular audits for checking compliance and quality assurance so that the product does not have any adverse effects when applied to the patients.
- Medical education and training amongst subjects
The role of the medical affairs management function is to educate health care professionals through the delivery of precise, complete and impartial information that supports a product. External education activities include seminars, small group meetings, updates and conferences etc. A sales representative also plays a reasonable role in providing doctors with recent updated information about the products and guidelines. So, sales representatives are also required to be trained to stay abreast with the latest information on the products, and also industry regulations.
- Medical communications for better quality of care
Field of medicine is ever-changing with constant flow of new knowledge and information. One of the main responsibilities is supplying the medical profession with the best available information pertaining to their area of interest for better clinical studies. Medical writing has established as an important function of medical affairs management, because it requires specialized knowledge and skills to write scientific documents which are well-structured, and presented in a clear and lucid manner to the patients.
WorkSure® Medical Affairs Management for improvement in patient outcomes
WorkSure® Medical Affairs Management provides consulting services to sales and marketing, clinical trials, pharmacovigilance, regulatory, and legal departments. We at WorkSure® are proactive at all levels, identify novel approaches and establish dedicated boundary-spanning teams for better clinical studies which will benefit the patients. We work at all stages in the life cycle of a biopharmaceutical product/device so that it reaches the patient without having any harmful effects.