Medical Affairs: Insights on Its Vitality and Pathways to Success
Medical Affairs (MA) teams in pharmaceutical firms work to inform healthcare providers, payers, administrators, and other stakeholders on the therapeutic benefits of novel medications. Their ability to bridge the gap between R&D and commercial/marketing operations, along with their managerial skills and scientific and research experience, allows them to do this.
The Medical Affairs function will probably continue to play a crucial role in generating the greatest possible science-driven discourse and, consequently, guaranteeing the alignment of shared aims to better patient outcomes, together with colleagues in the R&D and commercial or marketing activities.
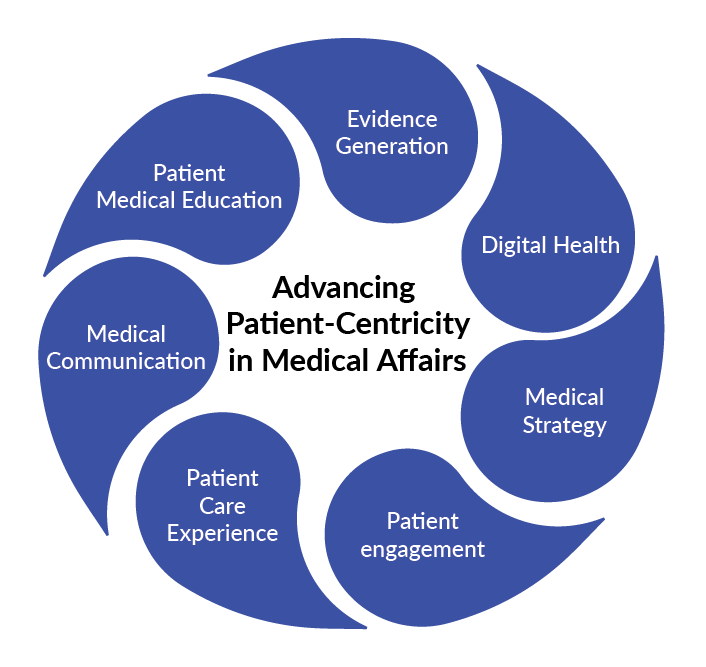
The primary responsibility of the local Medical Affairs team remains to interpret and contextualize the therapeutic value of pharmaceuticals for relevant stakeholders within the health care ecosystem. However, advances in biology and technology have drawn increased attention to outside-in concepts.
The following categories are now the emphasis of the Medical Affairs deliverables:
- Participation in and cooperation with clinical research.
- Clinical insight management, which includes administration, execution, and data collection.
- Generating Real-World Evidence (RWE) from various Real-World Data (RWD) sources, such as national registrations, regional databases, and other sources.
- Development of clinical dossiers and evaluations of health technologies.
- Clinical data analysis, publication, and distribution.
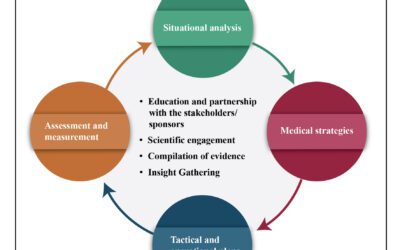
In addition to overseeing international scientific knowledge, Medical Affairs acts as a liaison between the organization, key opinion leaders (KOLs), and health care professionals (HCPs) through impartial, unbiased scientific communication. The timing and prioritization of medical activities can be influenced by a variety of circumstances, which will ultimately determine how best to develop and introduce a novel treatment after receiving local, regulatory permission and payment.
Future Medical Affairs professionals will mostly need to possess the following competencies:
- Ongoing superior understanding of recent developments in clinical research.
- Data science talents, such as research design, data analytic abilities, analysis and identification of clinical data gaps, and strategic data release and dissemination.
- A thorough grasp of the health care system.
- The capacity to create and implement long-term initiatives and health care solutions.
- The readiness to cooperate and engage with a variety of stakeholders within the dynamic health care ecosystem.
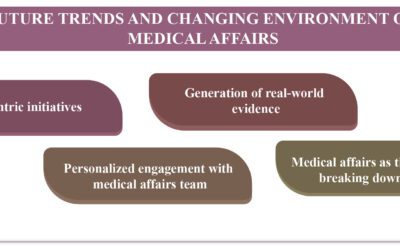
The future of Market Access in pharmaceutical companies will focus on patient-centricity, advanced technologies, RWD utilization, increased use of RWE, enhanced collaboration with new partners, and more regulatory scrutiny. Competency development in Market Access teams is crucial for meeting expectations and maximizing opportunities through an “outside-in” strategic approach. Companies are expanding their MA staffing to include experts in Biomedicine, Molecular Science, Pharmaceutical sciences, and Biochemistry. Advanced capabilities in data generation planning and engagement with healthcare professionals through various channels are now a priority. Market Access is also working on optimizing patient diagnostics, treatment initiation, and follow-up through collaboration with healthcare professionals and patients, as well as partnering with other pharmaceutical companies on disease awareness and innovative solutions like RWE and digital tools.
Conclusion
By comprehending patients, physicians, and worldwide market trends, Global Medical Affairs plays a vital role in developing creative and scientific value propositions for a company’s products. Based on insights gathered from patient and healthcare professional engagements, it outlines the medical and value-proven processes and propels the development of evidence to improve patient health outcomes. Global MA guarantees efficient communication between payers, regulators, healthcare providers, and patients. For novel medications to be introduced and successfully maintained in local markets after launch, strong coordination between global and regional MA teams is important. MA continues to play a crucial role in communicating with internal and external stakeholders and sharing knowledge pertinent to clinical practice in order to continuously improve patient outcomes.
At WorkSure®, we offer tailored solutions for MA, literature review, gap analysis, medical education and training, medico-marketing and business development, KOL management and engagement, medical communication, and more.
Summary of the article
Medical Affairs (MA) teams in pharmaceutical companies bridge the gap between R&D and commercial operations, ensuring healthcare stakeholders understand the therapeutic benefits of new medications. MA’s role is increasingly vital in advancing science-driven discussions to enhance patient outcomes. Key responsibilities include clinical research collaboration, managing clinical insights, and generating Real-World Evidence (RWE) from diverse data sources. MA also oversees clinical data analysis and distribution while liaising with key opinion leaders (KOLs) and healthcare professionals (HCPs).
WorkSure® provides comprehensive services in Medical Affairs, including tailored literature reviews, gap analysis, and medical communication strategies, ensuring optimal stakeholder engagement and knowledge dissemination.
To excel in the evolving landscape, future MA professionals must stay updated on clinical research, possess data science skills, and understand healthcare systems deeply. WorkSure® offers expert support in MA, helping companies meet these challenges through innovative solutions like medical education and medico-marketing.