Clinical Trials in India: The Booming Industry in India
Clinical trials are medical research conducted on humans that is planned to assess the efficiency of a drug or treatment and add to medical information. Some trials involve healthy members of the public, and others involve patients who may be offered the option of taking part in a trial during their care and treatment.
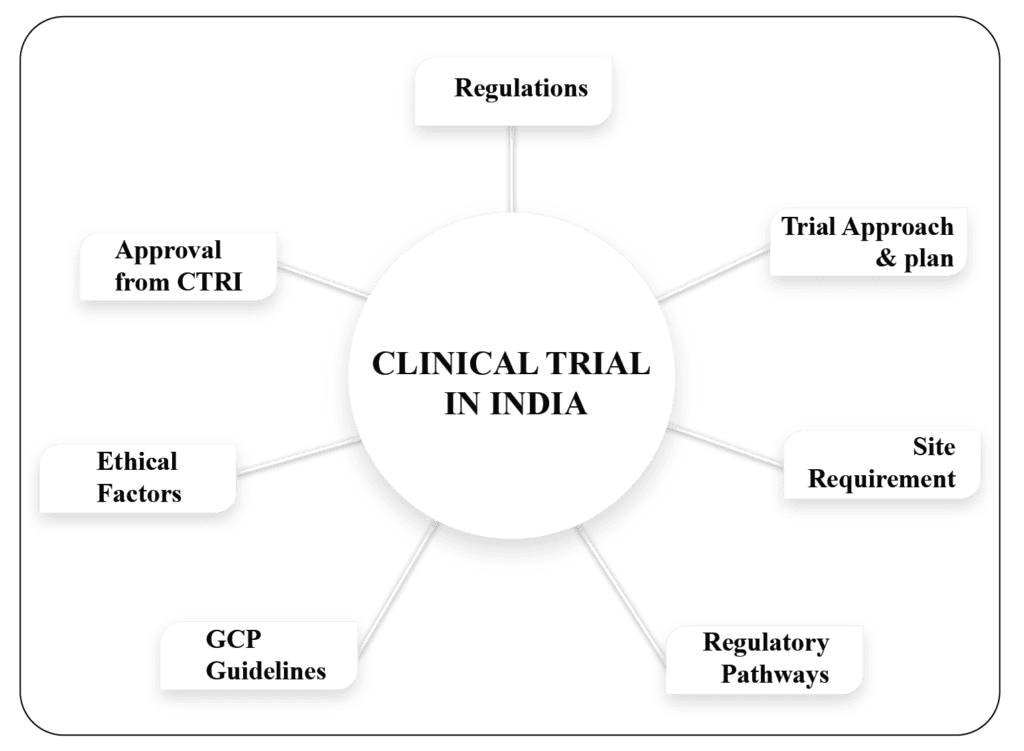
Over the past decade, India has become an important destination for many international pharmaceutical companies looking to carry out Clinical Research and Clinical Trials Management. Cost-effective trial strategies, a simplified Indian Regulatory system, trained manpower in medicine and clinical research, rich demographic diversity, vast patient pool, experienced scientists, doctors, physicians, and expert IT professionals have provided a great support and make India a prime place to do clinical trials. A total of 54,547 clinical trials are currently registered and ongoing in India as per Clinical Trial Registry India (CTRI) data. The Indian clinical trial market was estimated to be reached a valuation of USD 1.55 Billion in 2023. The market size is expected to reach USD 3.88 billion by 2030.
Clinical trials cover a wide range of different types of research. For example, trials are often used to test new medicines or vaccines, but they can also be used to look at new combinations of existing medicines. They can also be used to test whether giving a treatment in a different way will make it more effective or reduce any side effects. Some trials are designed to try out ways to prevent a particular disease in people who have never had the disease, or to keep a disease from rebounding.
Many different types of organization support clinical trials like pharmaceutical companies, charities etc. All trials are checked and monitored in similar ways to make sure that the rights of participants are protected. Each trial also has a sponsor who is responsible for running the trial. The sponsor may be the organization funding the trial or the institution hosting the research.
Many of these organizations involve patients to help decide what will be researched in the future. It is essential that research takes account of the needs and interests of the people it is trying to help. Specialists often know what needs to be found out about diagnosis and treatment, but patients and their families may think other aspects of care need further research.
Clinical trials are carried out in two stages – an early stage and a later stage. Early stage trials usually involve a small number of patients or healthy people. When psychological treatments or educational programmes are being tested, these early stage studies can be used to fine tune the treatment before it is tested on a large group of people. For trials of medicines and other treatments, early stage studies are carried out on a small group of people to assess safety by looking for unwanted side effects.
Later stage clinical trials involve larger numbers of participants and are usually randomized trials.
What happens if something goes wrong?
Before any trial can start, arrangements have to be put in place in case something goes wrong and people are harmed. Research ethics committees can refuse to allow a trial if it has no insurance or other compensation arrangement.
Pharmaceutical companies are insured so that if a patient is harmed by their drug, compensation can be paid. However, it is rare for patients to be seriously harmed by trial treatments, although some may cause unpleasant side effects. Trials funded by other organizations may not have this kind of insurance, but a payment may be made if something does go wrong.
WorkSure® in India provides services for preparation of Clinical Trial applications and other regulatory documents for clinical trial and drug marketing approval to such organizations. We are the pioneer in this industry and our trained workforce understands the clients need. We also provide technical expert representation to undertake discussions with regulatory authority.