Clinical Trial in India: Exploring Innovative Therapies
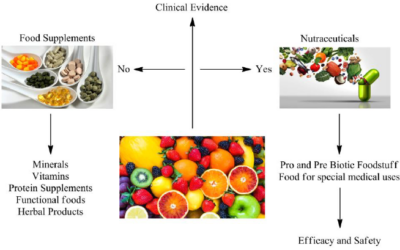
Clinical trial plays a vital role in evaluating the safety and effectiveness of nutraceutical products. Nutraceuticals, the fusion of nutrition and pharmaceuticals, are gaining popularity as natural compounds that offer health benefits and disease prevention. They also provide a crucial avenue for validating these innovative therapies, exploring their potential benefits, and establishing evidence-based guidelines. In recent years, India has emerged as a prominent destination for conducting nutraceutical clinical trials. The growth of nutraceutical clinical trials in India is expected to continue in the coming years.
The regulatory landscape for nutraceuticals in India is experiencing significant progress at present. The establishment of the Food Safety and Standards Authority of India (FSSAI) and the implementation of regulations on functional food and nutraceuticals have laid a robust foundation for the nutraceutical industry in the country. With advancements in scientific research, an increased focus on preventive healthcare, and the rising demand for evidence-based nutraceutical products, India presents a promising landscape for further exploration and development.
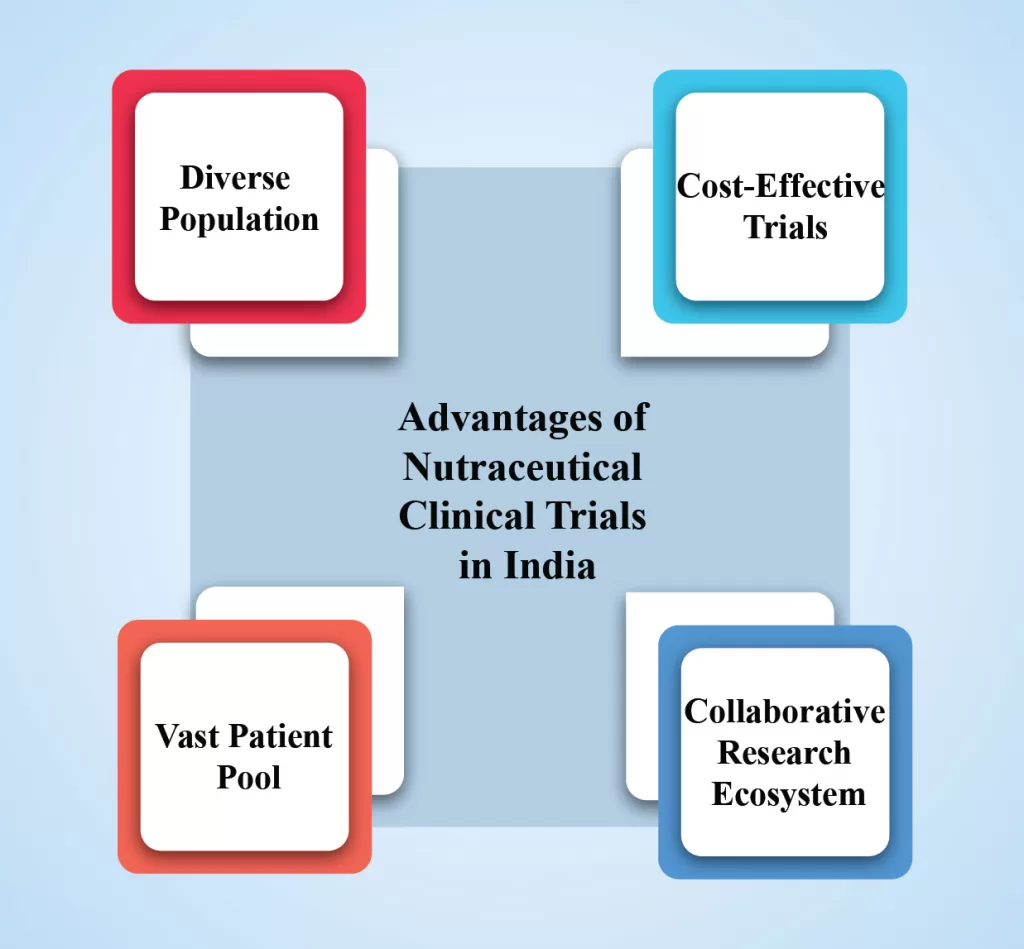
Advantages of Nutraceutical Clinical Trials in India:
- Diverse Population: India is known for its diverse population, which provides a broad spectrum of genetic variations and disease profiles. This diversity enhances the representativeness of study populations, allowing for more robust and reliable trial results.
- Cost-Effective Trials: Clinical trials conducted in India often have lower costs compared to Western countries, making it an attractive option for nutraceutical companies with budget constraints. The availability of skilled healthcare professionals and cost-effective infrastructure further contribute to the affordability of trials.
- Vast Patient Pool: India’s large population offers a significant advantage when recruiting participants for nutraceutical clinical trials. With a high prevalence of various health conditions, researchers can easily find suitable participants for specific study requirements.
- Collaborative Research Ecosystem: India has a thriving research ecosystem with numerous academic institutions, research centers, and contract research organizations (CROs) specializing in nutraceutical trials. Collaboration between industry, academia, and government bodies facilitates the smooth conduct of trials and ensures access to the latest scientific knowledge.
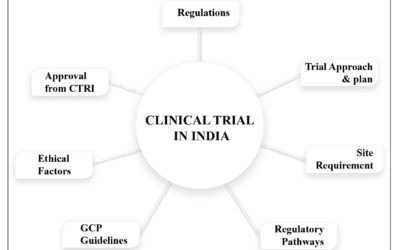
Regulatory Landscape for Nutraceutical Clinical Trials in India:
- Central Drugs Standard Control Organization (CDSCO): The CDSCO is the regulatory authority in India responsible for approving and overseeing clinical trials. It ensures adherence to ethical guidelines, patient safety, and data integrity. Nutraceutical clinical trials follow the same regulatory pathway as pharmaceutical trials, ensuring robustness and reliability in the trial process.
- Ethical Considerations: Nutraceutical trials in India follow both domestic regulations and international standards, ensuring compliance with global scientific and ethical benchmarks, including obtaining informed consent, protecting participant rights, and ensuring privacy and confidentiality. This promotes the acceptance and recognition of trial results in the international scientific community.
- Data Integrity and Quality: Regulatory authorities in India prioritize data integrity and quality. Trial sponsors must adhere to good clinical practices (GCP) guidelines, ensuring that trials are conducted ethically, data is accurately collected, and results are reliably reported.
- Streamlined Approval Process: India has implemented an expedited approval process for clinical trials, including nutraceutical trials. It involves approval from the Ethics Committee and CDSCO. This process minimizes delays and allows trials to commence quickly, saving time for sponsors.
Conclusion:
India has emerged as a favorable destination for conducting nutraceutical clinical trials due to its diverse population, cost-effectiveness, large patient pool, and collaborative research ecosystem. The country’s regulatory framework ensures ethical conduct, data integrity, and patient safety. As the demand for nutraceutical products grows, India’s role in advancing scientific knowledge and validating the effectiveness of these products through clinical trials becomes increasingly significant. By leveraging its strengths, India is unlocking the potential of nutraceuticals, ultimately contributing to improved healthcare outcomes worldwide.
The rapid expansion of the natural product and dietary supplements industry demands the backing of clinical trials to ensure both safety and effectiveness. At Worksure®, we offer comprehensive support throughout your clinical trial journey, enabling you to establish a strong presence in the dietary supplement market, expand your market size, and foster product differentiation. Worksure® specializes in designing and conducting clinical trials for nutraceuticals and herbal products. With expertise in both complementary and alternative medicine (CAM) and clinical pharmacology, Worksure® can bridge the gap between traditional knowledge and modern clinical research protocols. Our team of experienced professionals is dedicated to understanding your specific needs and delivering tailored solutions that meet regulatory standards.