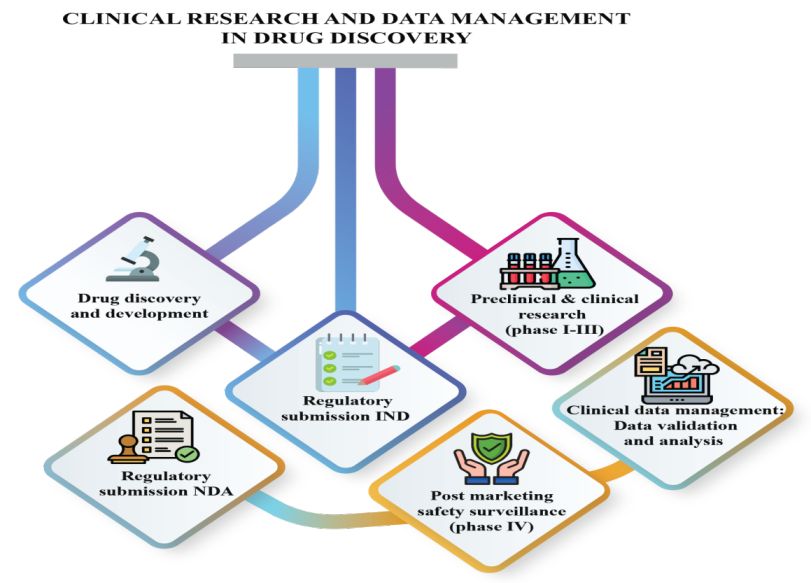
Clinical Research and Data Management: A Comprehensive Approach to Drug Discovery
Clinical research sponsors and pharmaceutical companies encounter numerous obstacles when seeking new drug approvals, often navigating complex regulations and stringent requirements. The clinical research sponsor requires a high-quality data report for getting new drug approval from Food and Drug Administration for their medical products. Clinical research data are important for the drug and medical device development processing. As soon as a potential moiety is identified it is subjected to pre-clinical studies for determination of safety, efficacy, pharmacokinetic and pharmacodynamic profiles of molecule which are later extrapolated to human volunteers or clinical subjects under clinical research. Clinical research is carried out meticulously at academic blocks, healthcare organizations and research study centers affiliated by regulatory bodies.
Clinical research focuses on improving knowledge of diseases, developing diagnostic methods and new/improved treatments or medical devices to make sure better patient care. Clinical research, in a systematic and orderly manner investigates the biological properties of investigational product in humans through various steps/phases, traditionally phase I-IV and seldom involving phase 0 and V also.
It produces concrete, objective data which forms the basis of risk-benefit analysis of the investigational drug and its fate. Evidences produced by clinical trials affect the decision making of clinicians in therapeutics thus lay down the foundation of evidence based medicine. Therefore, clinical research is a crucial part in drug discovery process and so is the clinical data management.
Clinical Data refers to the comprehensive statistical data of a drug/device/biological material, produced methodically from its conception in the lab to its introduction to the consumer market and thereafter. Data analysis involves the process where statistical data produced from clinical study as well as which is generated during data management reviews is studied to reach upon a conclusion on safety and efficacy profile of the candidate drug. The analyses are performed using listings, tables, figures with the assistance of specially designed software to suit the needs. In view of complexity of trial designs, huge magnitude of data generated, stringent timelines and corporate competition, an expert management of clinical data is mandatory for commercial success of the organization.
Considering its complications and the amount of time invested, entire process of clinical research is progressively relying on electronic skills. Clinical Trial Management System (CTMS) is such an electronic system which maintains and manages the planning, preparation, performance, and reporting of clinical trials. Similarly, Clinical Data Management System (CDMS) assists the researchers in the management of clinical data and its correlation to statistical investigations.
Clinical Data Management (CDM) is required in early data collection process and works on the development of data collection tools based on the clinical trial protocol. It ensures the precision and consistency of data collected throughout the trial process. CDM system is classified into paper based and electronic data capture system: Paper-based system and Electronic data capturing system. The former involves manual filing of Case report forms, the data capture aid at trial site which are mailed to the sponsor thereafter. In Electronic data capturing system, investigators at trial site directly upload the data on CDMS which is then processed to the data validation staff. To meet the fast growing and demanding pharmaceutical research, clinical data management system has evolved over the time.
Conclusion
Clinical research is the backbone of the drug discovery process, as is clinical data management for clinical research. The data management process ensures the success of a molecule in clinical trials through safety and efficacy analysis. It plays a key role in conducting, managing, and reporting clinical trials. Therefore, both clinical research and clinical data management form the foundation of faster and more successful drug development.
WorkSure®, a medical affairs and contract research organization, offers comprehensive solutions for medical writing, clinical research, and clinical data management. We serve pharmaceutical companies, biotech firms, hospitals, clinical research organizations, diagnostic companies, and health communication agencies, ensuring timely submissions and high client satisfaction.