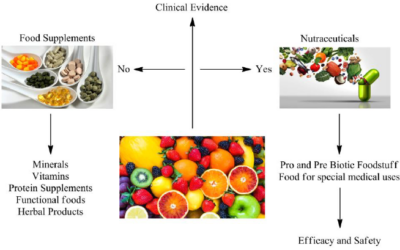
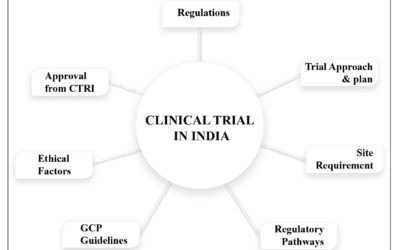
Clinical Research and Clinical Trials Management
Clinical Research and Clinical Trials Management:
Contract research organization, sometimes also called as Clinical research organization (CRO) is an organization that serves pharmaceutical, biotechnology, medical companies and various consumer healthcare industries in the field of clinical research. CRO provides support in new drugs and/or medical devices development that includes pre-clinical development to post marketing research activities. International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use (ICH-GCP) (E6 1.20) defines a CRO as: “A person or an organization (commercial, academic, or other) contracted by the sponsor to perform one or more of a sponsor’s trial-related duties and functions”. Depending on the nature of the clinical trial, selected CRO company will responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
Clinical Research Organizations have a large team of people with diverse skill sets and experience who are specialized in conducting time bound and cost effective research for sponsor companies. Sometimes, sponsor shire CROs when they do not have sufficient knowledge of regulatory affairs of any country. CROs have the capacity to conduct the day-to-day research activities that are either not possible or too expensive for a sponsor to achieve in-house. CROs are experts in the space, skill sets and active partners in clinical research.
Functional area of CROs encompass pre & post Clinical Research and Clinical Data Management, clinical research, clinical trials management, and pharmacovigilance, Medical writing, Data Management, Biostatistics and Training & Development. In order to accomplish these sponsor and CROs, and the sponsor and clinical site have different types of agreements. Moreover, CROs and a clinical site have to follow different guidance/regulations laid down by various regulatory authorities to accomplish their research. One of the major guidelines that must be followed by contract research organization is the ICH GCP. In addition to sponsor companies, CROs also assists various research institutions, and universities, NGOs in addition to governmental organizations (such as the NIH, EMEA, etc.).
The Clinical Trial Management is the essential set of tools to effectively plan, manage and track clinical study portfolio. It is used to manage all of the activities related to the setup, conduction, and closeout of clinical trials, including planning, preparation, tracking, monitoring, compliance, and reporting. Many clinical trial management systems are user friendly web-based, making them easy to use across multiple sites with a large group of medico and non medico users. A clinical trial management system makes clinical trial information more accessible and transparent for all. The study activities and financial health of clinical trials is streamlines by clinical trial management system. Hence, by using CTM clinical trial becomes more simplified, financial transparency is ensured, resources are optimized, and errors are prevented.
A first Medical Service provider in India, is a team of highly qualified and experienced professionals from apex institutes of India like AIIMS, PGI & IIT. We at WorkSure® work on three business models. Firstly as functional knowledge partners with dedicated team for medical writing, medical affairs, clinical-data-management/clinical data management, biostatistics, pharmacovigilance & training.
Secondly, we provide customized stand-alone services in pre-clinical & clinical scientific research. We provide medical writing services for preparation of safety reports like ICSR, PSUR. We provide statistical analysis of report/data and regulatory support solution. We provide regulatory solutions for preparation of Clinical Trial Application (CTA), Investigational Medicinal Product Dossier (IMPD), Investigational New Drug (IND) & New Drug Application (NDA), medical information services and KOL Management.
Thirdly, we have end to end solutions for medical writing, clinical research training and site development, clinical data management (CDM) and biostatistics for pre-clinical, medical and clinical research.
WorkSure® offers a specialized package for managing clinical data right from data management plan to its extraction for analysis and final reporting, with supportive statistical & medical writing services. With such a variegated umbrella of specialized services, WorkSure® is an emerging leader among Clinical Research Organizations setting new standards in this field.