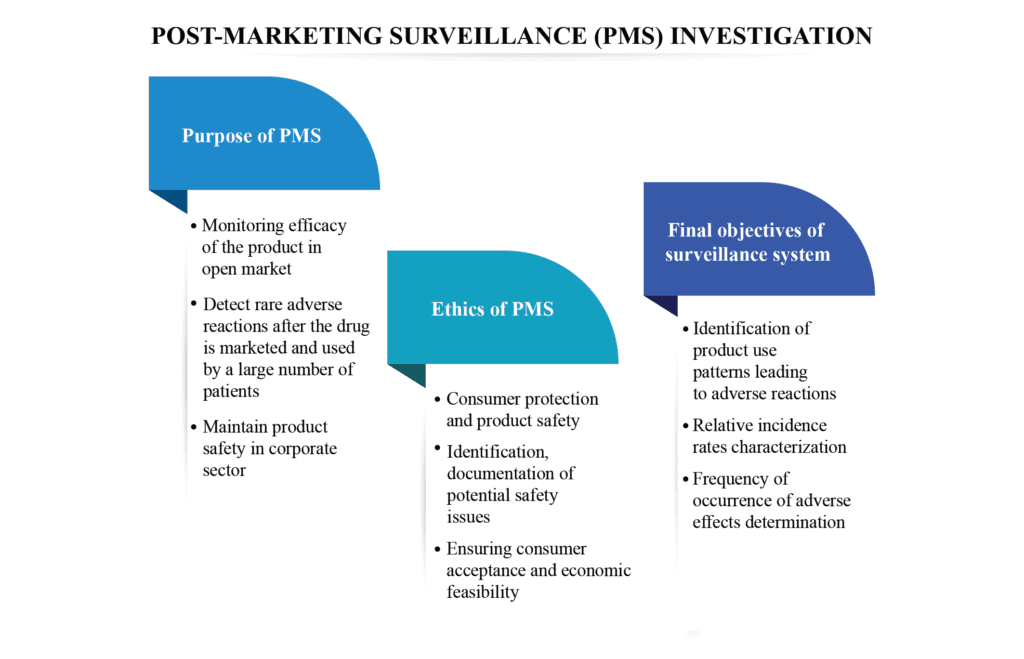
Post marketing surveillance (PMS) refers to monitoring of device/drug performance after successful completion of the clinical studies and the implementation of suitable action to improve patient safety. It is conducted after product is approved for sale by the European Union Medical Device Regulation (EUMDR) and the United States (US). It […]
Posts archive for July, 2023
Clinical Trials – Latest Technologies and Current Trends
Clinical Trials – Massive innovation was seen in clinical trials in the past couple of years, abating the fear of COVID-19 spread. Countless benefits from finding preventive measures, therapeutic drugs to developing vaccines have given the industry a more prominent audacity to take larger challenges. Not only the doctors and […]
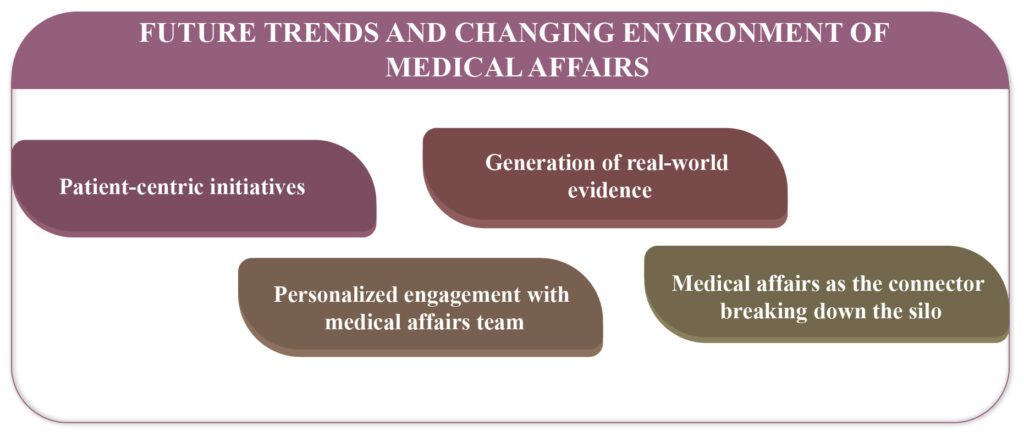
Medical Affairs – Future trends and changing environment
Medical Affairs (MA) focuses to find innovative solutions and continues to evolve fulfilling the needs of all healthcare providers and patients. Following recent trends have emerged in the life science industry. Patient-Centric Initiatives Emphasized patient-centricity that involves engaging the patient population, collaborating, and creating initiatives to satisfy the requirements of […]
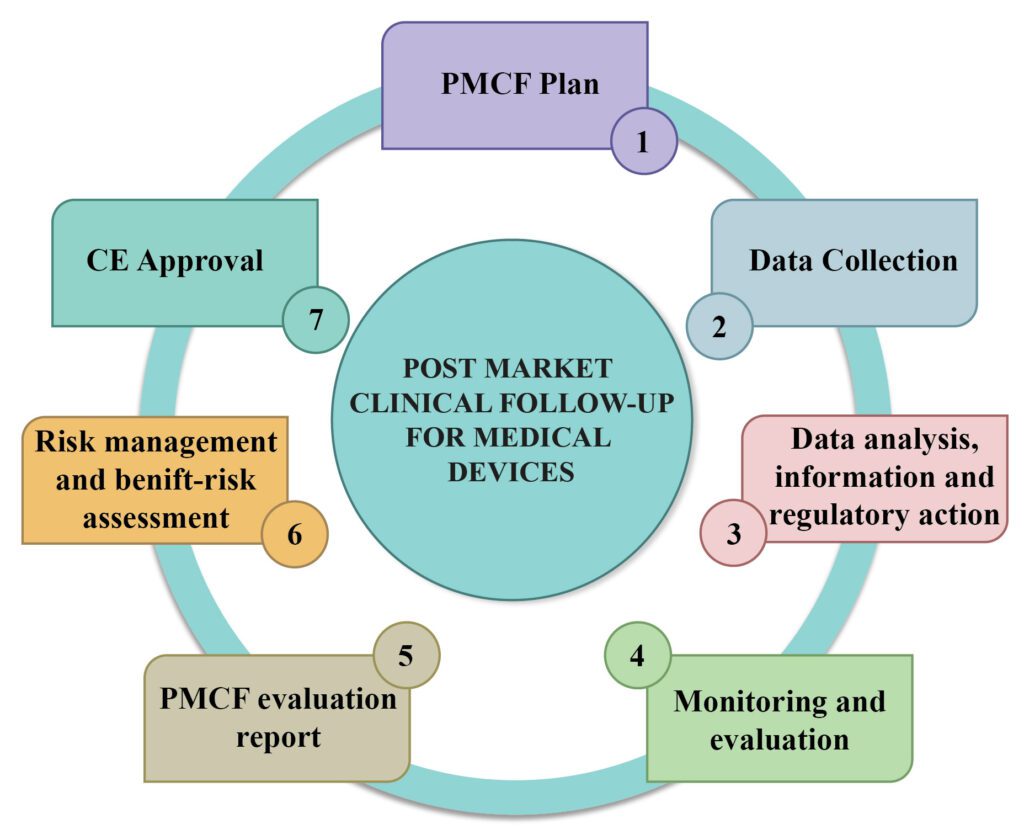
Post market clinical follow-up (PMCF) – An essentiality for Medical Devices
Post market clinical follow-up and Post-market surveillance (PMS) refers to the monitoring of medical devices after they have been cleared for sale and are in use by the public. One of the several types of post-marketing surveillance on the use of medical devices stipulated by the European Union Medical Device […]
Recent Posts
- Nutraceuticals in Clinical Trials: A Key to Evaluating Nutraceuticals and Dietary Supplements
- Medical Affairs: Insights on Its Vitality and Pathways to Success
- Clinical Research and Data Management: A Comprehensive Approach to Drug Discovery
- Education Information Management System (EIMS): A Step towards Improving the Quality of Education
- Medical Information Management System (MIMS): A Key to Better Healthcare Professional Engagement and Data Access
Archives
Categories
- Biostatistical Solution for HealthCare
- Clinical Data Management
- Clinical Study
- Clinical Trial
- Education Information Management System
- Electronic Data Management System
- Healthcare Communication
- KOL Management
- Medical Affairs
- Medical Communication
- Medical Writing
- Medico Marketing by WorkSure
- MIMS
- news
- Pharmacovigilance by WorkSure
- Sci-precis Survey Platform
- Uncategorized